The Role of Unconventional Hydrogen Bonds in Determining BII Propensities in B-DNA
Alexandra Balaceanu, Marco Pasi, Pablo D. Dans, Adam Hospital, Richard Lavery, and Modesto Orozco.
J. Phys. Chem. Lett., 2017, 8 (1), pp 21–2
DOI: 10.1021/acs.jpclett.6b02451
http://pubs.acs.org/doi/abs/10.1021/acs.jpclett.6b02451
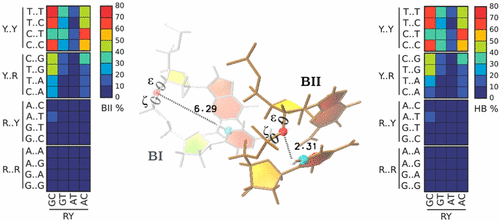
Abstract
An accurate understanding of DNA backbone transitions is likely to be the key for elucidating the puzzle of the intricate sequence-dependent mechanical properties that govern most of the biologically relevant functions of the double helix. One factor believed to be important in indirect recognition within protein–DNA complexes is the combined effect of two DNA backbone torsions (ε and ζ) which give rise to the well-known BI/BII conformational equilibrium. In this work we explain the sequence-dependent BII propensity observed in RpY steps (R = purine; Y = pyrimidine) at the tetranucleotide level with the help of a previously undetected C–H···O contact between atoms belonging to adjacent bases. Our results are supported by extensive multimicrosecond molecular dynamics simulations from the Ascona B-DNA Consortium, high-level quantum mechanical calculations, and data mining of the experimental structures deposited in the Protein Data Bank.

